Molekulare Psychokardiologie
AG Prof. Meyer

Major depression is a severe mental disorder, which manifests with cognitive-affective symptoms such as sadness, feelings of worthlessness and guilt, as well as physical symptoms including exhaustion, loss of energy and others. A growing body of evidence points to the crucial role of cytokines and inflammatory responses in the pathogenesis of depression. Cross-sectional studies have reported that patients with depressive symptoms exhibit higher levels of numerous circulating inflammatory markers, including interleukin-6 (IL-6), C-reactive protein and fibrinogen. Due to their substantial pleiotropy, cytokines elicit a broad range of diverse effects that may either be injurious or protective, depending on the particular cytokine, its concentration in the local tissue microenvironment, and the duration of its action. Interferons are probably the best characterized cytokines, as they are engaged in anti-viral response, inhibit proliferation and participate in immune surveillance and tumour suppression. Interestingly, treatment with interferon in viral hepatitis frequently induces depressive episodes, suggesting that this cytokine is also involved in the pathogenesis of depression.
Our focal points
Cytokines act on a variety of cells including neurons through the activation of signal transducers termed STATs (signal transducers and activators of transcription). Upon stimulation of cells with cytokines, STAT proteins are phosphorylated on a signature tyrosine residue in their carboxy-terminus and transiently accumulate in the nucleus. As their name implies, the STATs have the dual function of transducing signals from the plasma membrane to the nucleus, where they modulate gene expression. They are considered as key molecules in signal pathways that directly transmit extracellular signals from the plasma membrane to the nucleus without the interplay of second messengers. Originally discovered as components in interferon signalling, the STATs were later found to execute a plethora of cytokine-induced signals. STAT proteins constitute a family of cytokine-inducible transcription factors, which are involved in the regulation of broadly diverse biological processes, including cell growth, differentiation, immune regulation, development, apoptotic cell death and/or cell proliferation. In the human genome seven genes coding for different members of the STAT protein family have been identified so far.
Despite their functional diversity, all members of the STAT family share a conserved domain structure with an amino-terminal domain separated by a linker peptide from the core domain and a carboxy-terminal transactivating domain. The large core domain encompasses several structurally distinct domains beginning at the amino-terminal end with a four-helix bundle engaged in protein-protein interactions followed by a DNA-binding domain and a Src homology 2 (SH2) domain, which mediates receptor binding and dimer formation. Dimerization of phospho-STAT is achieved via reciprocal interaction between the phosphotyrosine of one monomer and the SH2 domain of the corresponding monomer. The carboxy-terminus is most divergent in size and sequence between the different members of the STAT family and in numerous splice variants the transactivating domain is deleted.
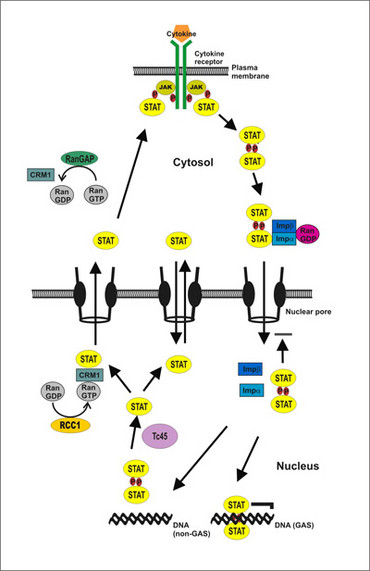
Upon binding of extracellular ligands to the cytokine receptor, non-covalently associated Janus kinases (JAKs) induce a cascade of phosphorylation steps that ultimately lead to the activation of STAT proteins (Figure 1). Activated JAK kinases, named after the Roman two-headed mythical god Janus, phosphorylate tyrosine residues within the cytoplasmic receptor tail, which serve as docking sites for the recruitment of cytoplasmic STAT molecules. Then JAK kinases phosphorylate STAT proteins on a single tyrosine residue in their carboxyl terminus. After entering the nucleus STAT dimers bind sequence-specifically to palindromic elements in the promoter region of cytokine-driven genes, thereby modulating transcriptional activity.
Previously, it was shown that STAT proteins are nucleo-cytoplasmic shuttling molecules that translocate between the cytoplasm and nucleus already in the absence of cytokine stimulation. Both phosphorylated and unphosphorylated STAT molecules enter the nucleus, albeit via different translocation pathways: a direct association of unphosphorylated STAT molecules with components of the nuclear pore complex called nucleoporins as well as binding of phospho-STAT dimers to import factors. Translocation of the unphosphorylated protein requires neither metabolic energy nor transport factors, but follows a concentration gradient, indicating that it functions as facilitated diffusion. In contrast to the constitutive shuttling of latent STAT1, nuclear import of phospho-STAT1 is energy-dependent and requires an intact RanGDP/RanGTP gradient across the nuclear envelope. The directionality of the active dimer transport is given by the asymmetric distribution of Ran nucleotide exchange factors across the nuclear envelope. Due to nucleotide hydrolysis by cytoplasmically localized RanGTPase-activating protein (RanGAP), the concentration of the guanosine triphosphate (GTP) form of Ran is low in the cytosol. In the nucleus, however, the guanine nucleotide exchange factor RCC1 maintains high levels of RanGTP, which promote the disassembly of the import complex and facilitate the binding of CRM1 to unphosphorylated STAT1. Both the nuclear import of activated STAT dimers via importins and export of unphosphorylated STATs via the export receptor chromosomal region maintenance 1 (CRM1) rely on the asymmetric distribution of the small GTPase Ran.
Tyrosine-phosphorylated STAT1 is unable to exit the nucleus before it has been dephosphorylated by Tc45 phosphatase. STAT1 dimers bound to GAS sites on DNA are effectively protected from dephosphorylation and stay longer in a transcriptionally active state. Thus, nucleocytoplasmic shuttling enables the coupling between receptor processes at the cell membrane and transcriptional regulation in the nucleus, because enzymatic dephosphorylation is proportional to the sequence-specific dissociation rate of STAT1 from DNA.
STAT1 has been implicated as key molecule in interferon-induced anti-viral defence and apoptotic cell death. Stimulation with interferon- gamma results in the formation of STAT1 homodimers that bind to GAS (gamma-activated site) elements in the promoter region of interferon-gamma-activated genes. In response to interferon-alpha , STAT1 primarily forms the transcription factor interferon-stimulated gene factor 3 (ISGF3), which also includes STAT2 and interferon-regulated factor-9 (IRF-9, also termed p48). ISGF3 binds to interferon-stimulated response elements (ISREs) and modulates the expression of genes engaged in anti-viral response. However, the precise molecular steps facilitating target gene recognition are not well understood. Using a mutational approach, we are focusing on molecular mechanisms both at the cellular and organismic level that efficiently convert cytokine signals into cellular and behavioural responses. Research on the molecular action of STAT proteins may help to gain a better insight into the complex pathophysiology of depressive disorders.
Acknowledgement: This work is and has been supported by grants from the Deutsche Forschungsgemeinschaft und Deutsche Krebshilfe to T.M..
Kontakt
Kontaktinformationen
- Telefon: +49 551 3964940
- E-Mail-Adresse: thomas.meyer(at)med.uni-goettingen.de
Das könnte Sie auch interessieren